- Home
- Company
- Products
- Status COVID-19/Flu A&B
- Status Flu A&B
- Status Strep A Flip Cassette Tests
- Indicaid COVID-19 Rapid Antigen Test
- QuickVue RSV Antigen Tests
- OraQuick HCV Rapid Antibody Test
- OraQuick Advance Rapid HIV-1/2 Antibody Test
- DUKES BioFlexx Gloves 200 pack
- PowerChek 2019-nCoV Real-time PCR Kit
- Specimen Collection and Transport Kit
- Contact Us
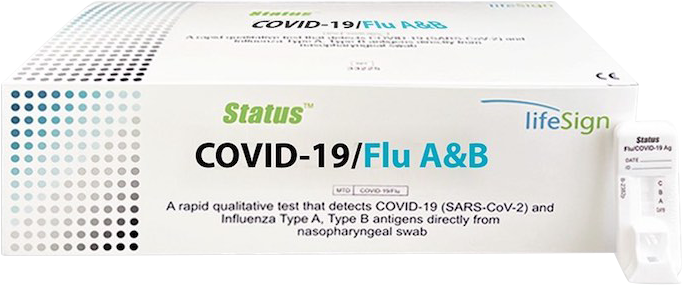
Status COVID-19/Flu A&B - Rapid SARS-CoV-2 / Influenza A+B Antigen Panel Test
A rapid, Point of Care immunochromatographic assay for the simultaneous qualitative detection and differentiation of nucleocapsid antigen from SARS-CoV-2, influenza A and influenza B directly from nasopharyngeal swab specimens obtained from individuals, who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider, within the first five days of onset of symptoms.
FDA Emergency Use Authorization for Use in CLIA Waived Settings
Product Features:
- Point-of-Care Rapid Test For 3 Viral Antigens From Just 1 Swab Sample
- Detects and Differentiates Between SARS-CoV-2, Influenza A and Influenza B Nucleocapsid Protein Antigen
- Broad Detection of Influenza A and Influenza B Strains, Including H1N1
- Sensitivity and Specificity
– COVID-19 – Sensitivity: 93.9%, Specificity: 100%
– Flu A – Sensitivity: 91.4%, Specificity: 95.7%
– Flu B – Sensitivity: 87.6%, Specificity: 95.9%
- Same superior performance for influenza A+B as BioSign® Flu A+B and Status™ Flu A&B
- Results in about 15 Minutes — Quick and Reliable
- Streamlined Procedure with Onboard Extraction — No Sample Transfer Required
- Pre-Measured, Unit Dose Reagent Capsule — No Counting Drops
- Easy-to-Read Visual Color Band Signal — No Reader Required
- Built-in Procedural Control
- Room Temperature Storage of Test Kit
Key to Simple Results
- Control line (C): a colored Control line indicates the test is working properly.
- Test line (T): a colored Test line (A, B or CoV19) indicates the test is POSITIVE (i.e., influenza A, B or COVID-19 positive).
- Sample well (S): Insert swab and perform onboard extraction.
